有機化學/烯烴
烯烴為不飽和烴,其結構特徵是分子中含有C=C雙鍵,公式為CnH2n
烯類命名
[編輯]E-Z 標示
[編輯]比較E-Z與cis-trans標示
[編輯]特性
[編輯]非鏡像異構(Diastereomerism)
[編輯]限制轉動
[編輯]相對穩定度
[編輯]反應
[編輯]製備
[編輯]製備烯烴有幾種不同方式。下面有的方式(像是威悌反應)我們這邊只簡短介紹,後面會更詳細的說明。
目前只需要知道這些是製備烯的方式就好。
鹵代烷(Haloalkanes)的脫鹵化氫(Dehydrohalogenation)
[編輯]
鄰二鹵化物(Vicinal Dihalides)的脫鹵(Dehalogenation)
[編輯]醇類脫水
[編輯]炔類還原
[編輯]威悌反應(Wittig Reaction)
[編輯]威悌反應是醛酮類與三苯基磷鎓內鹽(威悌試劑)作用生成烯烴和三苯基氧膦的一類有機化學反應
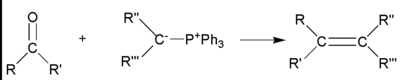
馬爾科夫尼科夫規則(Markovnikov's Rule)
[編輯]Before we continue discussing reactions, we need to take a detour and discuss a subject that's very important in Alkene reactions, "Markovnikov's Rule." This is a simple rule stated by the Russian Vladmir Markovnikov in 1869, as he was showing the orientation of addition of HBr to alkenes.
His rule states:"When an unsymmetrical alkene reacts with a hydrogen halide to give an alkyl halide, the hydrogen adds to the carbon of the alkene that has the greater number of hydrogen substituents, and the halogen to the carbon of the alkene with the fewer number of hydrogen substituents" (This rule is often compared to the phrase: "The rich get richer and the poor get poorer." Aka, the Carbon with the most Hydrogens gets another Hydrogen and the one with the least Hydrogens gets the halogen)
This means that the nucleophile of the electophile-nucleophile pair is bonded to the position most stable for a carbocation, or partial positive charge in the case of a transition state.
例子
[編輯]馬氏規則產物
[編輯]馬氏規則加成
[編輯]反馬氏規則加成
[編輯]為什麼馬氏規則可行?
[編輯]規則例外
[編輯]加成反應
[編輯]硼氫化(Hydroboration)
[編輯]硼氫化/氧化
[編輯]立體化學和定向
[編輯]汞氧化/去汞(Oxymercuration/Reduction)
[編輯]狄耳士–阿德爾反應(Diels-Alder Reaction)
[編輯]催化加氫(Catalytic addition of hydrogen)
[編輯]親電加成(Electrophilic addition)
[編輯]鹵化(Halogenation)
[編輯]氫鹵化(Hydrohalogenation)
[編輯]氧化
[編輯]聚合
[編輯]取代和消除反應機制
[編輯]親核取代反應
[編輯]備註
[編輯]SN1 vs SN2
[編輯]SN2 反應
[編輯]Reactivity Due to Structure of SN2
[編輯]親核性(Nucleophilicity)
[編輯]List of descending nucleophilicities
[編輯]離去基
[編輯]離去基的相對反應性
[編輯]溶劑
[編輯]Relative Reactivity of Solvents
[編輯]SN1 反應
[編輯]Reactivity Due to Structure of SN1
[編輯]溶劑
[編輯]摘要
[編輯]消除反應(Elimination Reactions)
[編輯]Note
[編輯]E1 vs E2
[編輯]反應速率
[編輯]查依采夫規則(Zaitsev's Rule)
[編輯]查依采夫規則,也稱作扎伊采夫規則或扎伊切夫規則,是指醇或鹵代烴在進行消除反應時,主要生成物會是碳碳雙鍵上取代基較多的烯烴(最穩定的烯烴)。
例如,2-丁醇進行消除反應時,主要生成物是2-丁烯,1-丁烯只是次要生成物。

