有机化学/二烯烃
二烯烃指含有分子中含有两个碳碳双键的有机化合物。
二烯烃种类
[编辑]孤立二烯烃(Isolated Dienes)
[编辑]
孤立二烯烃为两个双键相隔两个以上单键的二烯烃。
孤立二烯烃的双键距离太晚,一般不会有特殊的反应。可以视为比较大的烯烃来判断反应。
累积二烯烃(Cumulated Dienes)
[编辑]
累积二烯烃为两个双键相邻的二烯烃。最简单的形式为丙二烯。
Cumulated dienes are typically less stable than other alkenes. The main reason for the instability is the fact that this sort of diene is a probable transition state for an alkyne's triple bond to move down the carbon chain towards the most stable position. As you may recall, rotation does not occur around any π-bond, which means that cumulated double bonds can lead to a less stable, higher energy compound being formed.
Typically, cumulated dienes are discussed only in advanced courses in organic chemistry, and so they will not be discussed in detail here. Beginning organic chemistry students should merely remember that cumulated dienes are 1) high energy and 2) most likely found as transition states.
共轭二烯烃(Conjugated Dienes)
[编辑]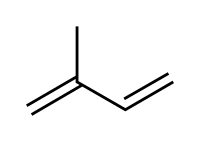
共轭二烯烃为两个双键中间恰有一个单键的二烯烃。
Conjugated dienes are dienes which have at least two double bonds separated by a single carbon-carbon bond, and for this reason conjugated dienes are observed to have a special stability due to the overlap of electron orbitals. The areas of concentration of negative charge (electron density) overlap across the three bonds (two double bonds and one single bond) forming what behaves essentially as a single, continuous π-bond across three carbon atoms. This delocalization of electron density stabilizes the molecule, resulting in the arrangement of lowest energy.
Atoms other than carbon which are capable of multiple bonds may also participate in conjugation. The heteroatoms most often associated with conjugation in dienes and other molecules are nitrogen and oxygen, but theoretically the majority of atoms in the Periodic Table of Elements could participate in conjugation chemistry. Most often, in organic molecules, heteroatoms participating in conjugation will be nitrogen atoms within a ring structure or a double-bonded oxygen attached to form a ketone or aldehyde.
Conjugation of double bonds is the largest part of what makes aromaticity relevant in organic chemistry, and conjugated double bonds have many other significant impacts on other types of dienes as well.
共轭
[编辑]双键的共轭
[编辑]二烯烃特性与反应
[编辑]Common Reactions of Conjugated Dienes
[编辑]氢溴化
[编辑]狄耳士-阿德尔反应(Diels-Alder Reaction)
[编辑]狄耳士–阿德尔反应是一种环加成反应,共轭双烯与取代烯烃(一般称为亲双烯体)反应生成取代环己烯。
狄尔斯-阿尔德反应有如下规律:
1、区域选择性:反应产物往往以“假邻对位”产物为主。即若把六元环产物比作苯环,那么环上官能团(假设有两个官能团)之间的相互位置以邻位(如1),或者对位为主(如3)。

2、立体选择性:反应产物以“内型(即5)”为主,即反应主产物是经过“内型”过渡态得到的。

3、立体专一性:加热条件下反应产物以“顺旋”产物为唯一产物;光照条件下以“对旋”产物为唯一产物。
比如以下两个热反应中,产物7、8的相对立体构型都是唯一的,两个烯烃原料原有的官能团A,B,C,D的顺反立体化学关系,都在产物中得到忠实地翻译。

